2,5-dimethylpyrrole

1. Product Introduction
Product Name: 2,5-Dimethylpyrrole ; 2,5-Dimethylpyrrole
Abbreviation/Abbr.: DMPY
Chemical Registration Number/CAS No.:625-84-3
Molecular Formula: C6H9N
Molecular weight: 95.14
Structural formula:
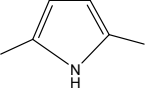
Properties and uses
Appearance: Colorless or yellow transparent liquid
Density: 0.935 g/cm³ at 20°C
Boiling Point: 165°C (740 mmHg)
Solubility: Insoluble in water, but soluble in organic solvents such as ethanol, DMF, and petroleum ether.
Application: Catalyst for the synthesis of 1-hexene, preparation of artificial urine and its biological detection.
1. Intermediates in drug synthesis
Pyridine ring is a very important structural unit in many bioactive molecules, which is called "dominant structure".
Construction of drug molecular skeleton: 2,5-dimethylpyrrole can be used as a starting material for the synthesis of more complex nitrogen-containing heterocyclic compounds, which are the core structures of many antibiotics, anti-inflammatory drugs, anti-tumor drugs and other drugs.
Functional derivatives: The carbon and nitrogen atoms on the pyrrole ring can be further chemically modified (such as sulfonation, acylation, condensation with aldehydes and ketones, etc.) to link other pharmacodynamic groups and construct candidate drug molecules with specific pharmacodynamic activity.
2. Synthesis of pyrrolidines and macrocyclic compounds
This is a very classical and important use of 2,5-dimethylpyrrole.
Porphyrin Precursor: As the core structure of vital biological compounds such as chlorophyll and heme, porphyrin is a fundamental building block. In laboratory synthesis of porphyrin and its analogs (e.g., porphyrin), 2,5-dimethylpyrrole serves as a key monomer. Through condensation reactions with other pyrrole derivatives, complex macrocyclic porphyrin systems can be constructed.
Crown ethers and cavity ethers: They can also be used to synthesize some nitrogen-containing macrocrown ethers or cavity ligands, which can specifically recognize and bind metal ions or organic cations, and have applications in analytical chemistry and separation science.
3. Coordination chemistry and metal complexes
The nitrogen atom on the pyrrole ring has a lone pair of electrons and can coordinate with the metal ion as a coordination point.
Metal complexing agents: 2,5-dimethylpyrrole can act as a ligand to form complexes with certain metal ions. Although its coordination ability is generally weaker than that of pyridine or amines, it can be used to simulate the coordination environment of natural porphyrin proteins or for catalytic studies in specific systems.
4. Materials science
It also has applications in materials science based on its electronic properties and role as a porphyrin synthesis precursor.
Organic photoelectric materials: Synthetic porphyrin compounds are crucial for organic solar cells, dye-sensitized solar cells, and photoelectric sensors. 2,5-Dimethylpyrrole, serving as a precursor for these porphyrin materials, is also used in these applications.
Metal-organic frameworks (MOFs): Porphyrin-based ligands synthesized from MOFs can be used to construct porphyrin-based MOFs, which exhibit excellent performance in gas adsorption, separation, and photocatalysis.
5. Chemical derivatization and protecting groups
2,5-dimethylpyrrole sometimes plays a special role in complex multi-step organic synthesis.
Amino protection: In some cases, its structure can be used to temporarily protect the amino group or as a steering intermediate for the synthesis of other heterocycles.
2. Quality Standards
| project /Item | metric /Specification | Method/Method | |
| 1 | Appearance | Colorless or pale yellow liquid colorless or light yellow liquid |
sense organ |
| 2 | Content/Assay,% | ≥99.5 | GC |
| 3 | Water,% | ≤0.2 | K-F Trace Moisture Determination |
| 4 | Ketone, ppm | ≤10 | GC |
| 5 | Heavy metal Pb, ppm |
≤5 | ICP |





