2-chloro-N,N-dimethylacetamide

1. Introduction to 2-chloro-N,N-dimethylacetamide
Product Name: 2-Chloro-N,N-Dimethylacetamide ; 2-Chloro-N, N-dimethylacetamide
Abbreviation: 2-Cl-DMAC
Chemical Registration Number/CAS No.:2675-89-0
Molecular Formula: C4H8ClNO
Molecular Weight: 121.57
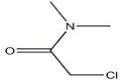
Structural Formula:
Properties and uses
This product is a colorless, transparent or light yellow liquid, with a strong irritating smell, has a strong corrosive effect on the skin.
This product is slightly soluble in water and readily soluble in organic solvents such as methanol, ethanol, and dichloromethane. It is relatively stable in air, but the color becomes gray and dull after prolonged storage.
Melting point: 15-16℃, boiling point: 98-100°C/ 11mmHg.
This compound serves as an intermediate in organic synthesis, used in the production of novel pharmaceuticals such as Camotis, KarXT, and Evenamide.
1. Pharmaceutical synthetic intermediates (core uses)
This is the most important and widespread application of 2-chloro-N,N-dimethylacetamide. It is used to construct the core structure of many important drugs.
Anticancer drug: This serves as a key intermediate in the synthesis of bendamustine. Bendamustine, a nitrogen mustard-type bifunctional alkylating agent, is used to treat chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma. The chlorine atom in the 2-chloro-N,N-dimethylacetamide structure is the critical component responsible for its alkylating action and DNA damage.
Neurological drugs: The compound can be used to synthesize the molecular skeleton of some sedative-hypnotic drugs and antidepressants. Its structure can be used as a link unit or to introduce specific pharmacophores.
Other drug molecules: As a universal alkylation reagent, it can be used to introduce the "-CH₂C(O)N(CH₃)₂" fragment in the structural modification and optimization of various drug molecules.
2. Pesticide synthetic intermediates
In the field of agrochemicals, heterocyclic compounds containing nitrogen and halogens are very common.
Herbicides and insecticides: 2-chloro-N,N-dimethylacetamide (2-CCA) serves as an intermediate for synthesizing certain amide-based herbicides or nitrogen-containing heterocyclic insecticides. Its high reactivity enables efficient coupling with other cyclic structures to create biologically active molecules.
3. Organic synthesis blocks
In the basic organic synthesis, it is a multifunctional synthase that can undergo two key reactions:
As an electrophilic reagent (alkylating agent): The chlorine atom acts as an excellent leaving group, endowing the methylene group (-CH₂-) in the molecule with strong electrophilicity. It can undergo nucleophilic substitution reactions with various nucleophiles (such as amines, alcohols, phenols, thiols, and carbanions), yielding a series of N,N-dimethylglutamide derivatives.
Participating in cyclization reactions: Due to its intramolecular dual functionality (containing both an electrophilic center (C-Cl) and a nucleophilic center (amido oxygen or nitrogen atoms under specific conditions)), it can efficiently react with bifunctional reagents to construct heterocyclic compounds such as oxazolidinones and morpholino. These heterocycles form the core structures of numerous natural products and pharmaceuticals.
4. Polymer chemistry
Monomer or modifier: Although uncommon, 2-chloro-N,N-dimethylacetamide can be used as a functional monomer in polymerization reactions or to modify existing polymer chains with side groups to introduce specific reaction sites or alter the physical and chemical properties of polymers.
In a word:
2-Chloro-N,N-dimethylacetamide is a vital intermediate in pharmaceutical and pesticide synthesis, particularly renowned for its irreplaceable role in the production of the anticancer drug bendamustine.
2. Quality standards
| project | metric | method |
| surface | Colorless or pale yellow liquid | sense organ |
| content ,% | ≥98.5 | GC |
| M str ,% | ≤1.5 | K-F determination of moisture |
| Monosodium (2,2-dichloro-N,N-dimethylacetamide),% | ≤0.7 | GC |
| Other impurities,% | ≤0.3 | GC |





